-
-
Guilin Pharmaceutical Co., Ltd.
SPAQ-CO® Disp
Common Name:Sulfadoxine/Pyrimethamine + Amodiaquine (Hydrochloride) Dispersible Tablets
This product passed the WHO prequalification in 2018 and is the world's first drug for malaria prophylaxis in children approved for seasonal malaria chemoprevention (SMC).
Therapeutic Area: prophylaxis for malaria.
-
-
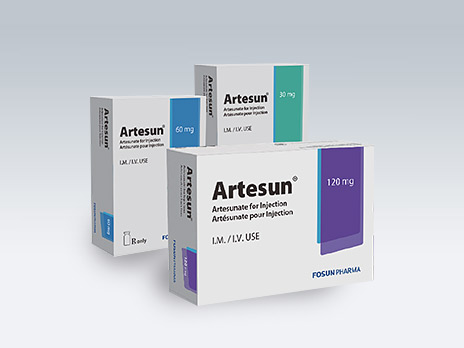
Guilin Pharmaceutical Co., Ltd.
Artesun®
Common Name:Artesunate for Injection
It is a global initiative and the "gold standard" for treatment of severe malaria. It passed the WHO prequalification in 2005, is the first-choice drug for severe malaria recommended by the WHO, and is known as an "anti-malarial miracle drug".
Therapeutic Area: treatment of severe malaria
-

-
Shenyang Hongqi Pharmaceutical Co., Ltd.
Pyrazinamide Tablets
Common Name:Pyrazinamide Tablets
First-line anti-TB drug indicated for the treatment of tuberculosis in combination with other anti-TB drugs (such as streptomycin, isoniazid, rifampicin and ethambutol). a product passed consistency evaluation.
-

-
Shenyang Hongqi Pharmaceutical Co., Ltd.
Ethambutol Hydrochloride Tablets
Common Name:Ethambutol Hydrochloride Tablets
First-line anti-tuberculosis drug indicated for the treatment of tuberculosis caused by mycobacterium tuberculosis in combination with other anti-tuberculosis drugs; also used for the treatment of tuberculous meningitis and atypical mycobacterial infections; a product passed consistency evaluation.
-
-
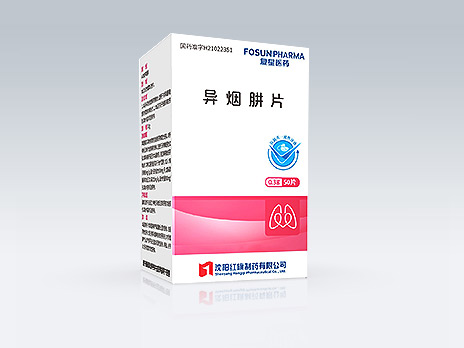
Shenyang Hongqi Pharmaceutical Co., Ltd.
Isoniazid Tablets
Common Name:Isoniazid Tablets
First-line antituberculosis drug indicated for the treatment of various types of TB, including tuberculous meningitis and other mycobacterium tuberculosis infections. The first isoniazid product passed consistency evaluation.
-
-
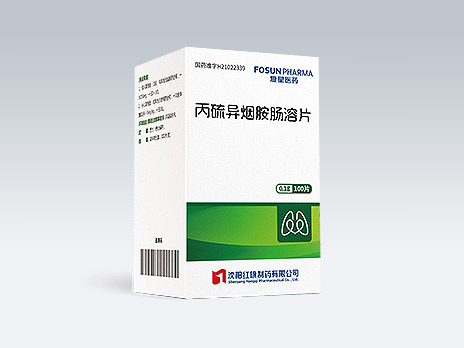
Shenyang Hongqi Pharmaceutical Co., Ltd.
Protionamide Enteric-coated Tablets
Common Name:Protionamide Enteric-coated Tablets
Second-line antituberculous drug and the essential therapeutic drug for treatment of drug-resistant tuberculosis by inhibiting the cell wall synthesis of mycobacterium tuberculosis; a required drug for short-course regimen for treatment of drug-resistant tuberculosis.
-
-
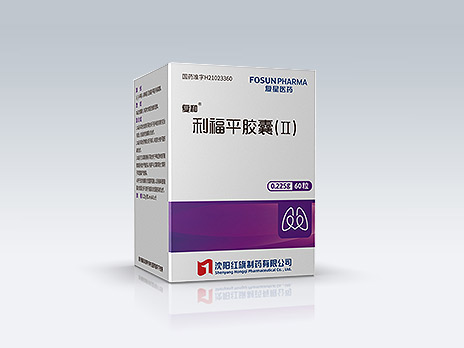
Shenyang Hongqi Pharmaceutical Co., Ltd.
Fu He
Common Name:Rifampicin Capsules (II)
First-line drug for treatment of drug-susceptible tuberculosis; rifampicin with less hepatic impairment and higher bioavailability; achieving the therapeutic effect of 0.6 g of general rifampicin with only two capsules per time.
-
-
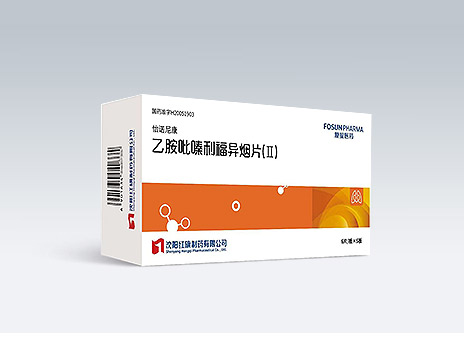
Shenyang Hongqi Pharmaceutical Co., Ltd.
Yi Nuo Ni Kang
Common Name:Ethambutol Hydrochloride, Pyrazinamide, Rifampicin and Isoniazid Tablets (II)
A drug recommended by WHO guidelines in standard treatment regimens for drug-susceptible tuberculosis; the only fixed-dose four-drug combination for tuberculosis treatment included in the WHO Model Lists of Essential Medicines; improved patient compliance; reduced drug resistance rate.
-
-
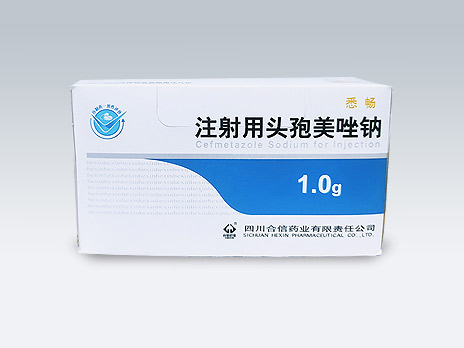
Sichuan Hexin Pharmaceutical Co., Ltd.
Xi Chang/Bi Li Shu
Common Name:Cefmetazole Sodium for Injection
It has quality of brand name drug, and is a product under National Medical Insurance Class B; a confidence choice for monotherapy of mixed infection of aerobic bacteria and anaerobic bacteria; simple choice for prevention of surgical infection and perioperative infection; economical choice for empirical therapy of infection of ESBLs-producing bacteria.
-
-
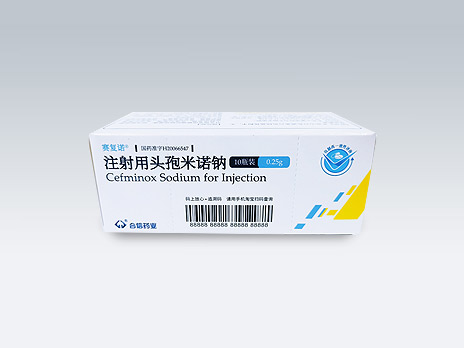
Sichuan Hexin Pharmaceutical Co., Ltd.
赛复诺
Common Name:Cefminox Sodium for Injection
It has quality of brand name drug, and is a product under National Medical Insurance Class B; it is recommended by many guidelines and consensus at home and abroad; it has a broad antibacterial spectrum, covering G+ bacteria, G-bacteria and anaerobic bacteria; it has good stability against ESBL enzymes, and has a low drug resistance rate clinically.
-
-
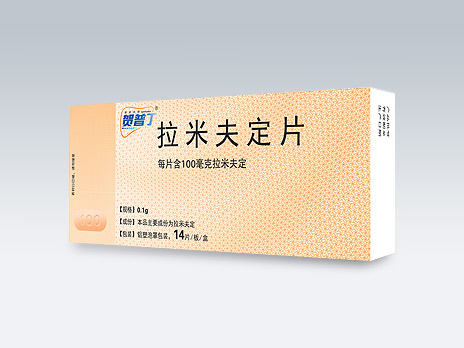
JSK (Suzhou) Pharmaceutical Co., Ltd.
Heptodin®
Common Name:Lamivudine Tablets
It is an original product under National Medical Insurance Class B; it is the first oral anti-HBV drug marketed in China; it has high safety, has strong antiviral ability, and is a classic drug for patients with chronic hepatitis B; long-term treatment with it can effectively improve liver tissue inflammation and delay the progress of chronic hepatitis B disease.
-

-
瑞塞宁
Common Name:Clindamycin Hydrochloride Capsules
It is a China's national essential drug under National Medical Insurance Class A, and is included in the National Centralized Drug Procurement List; it is recommended by multiple guidelines at home and abroad; it is an antibacterial drug for unrestricted use, and an experience choice for treatment of various common infections.
-
Our Company
Shanghai Fosun Pharmaceutical (Group) Co., Ltd. is a global innovation-driven pharmaceutical and healthcare industry group deep-rooted in China

- Fosun Pharma is patient-centered and clinical needs-oriented. The company continuously enriches its innovative product pipeline through independent research and development, cooperative development, license-in, and in-depth incubation. Fosun Pharma improves the research and clinical development capabilities of FIC (First-in-class) and BIC (Best-in-class) new drugs as well as accelerates the R&D and launch of innovative technologies and products.
-
Our Business
Shanghai Fosun Pharmaceutical (Group) Co., Ltd. (Fosun Pharma) directly operates businesses including pharmaceutical manufacturing, medical devices, medical diagnosis, and healthcare services. As a shareholder of Sinopharm Group Co., Ltd., Fosun Pharma expands its areas in the pharmaceutical distribution and retail business.

- Fosun Pharma is patient-centered and clinical needs-oriented. The company enriches its innovative product pipeline through diversified and multi-level cooperation models such as independent research and development, cooperative development, license-in, and in-depth incubation. Fosun Pharma has formed technological platforms for innovative small molecule drugs, antibody drugs, and cell therapy with a focus on key disease areas including oncology and immunomodulation, metabolism and digestive system, and central nervous system. Fosun Pharma also vigorously explores cutting-edge technologies, such as RNA, oncolytic viruses, gene therapy and PROTAC, to enhance its innovation capabilities.
-
R&D
Shanghai Fosun Pharmaceutical (Group) Co., Ltd. is a global innovation-driven pharmaceutical and healthcare industry group deep-rooted in China.

- Guided by innovation and internationalization, the company continuously enriches its innovative product pipeline through diversified and multi-level cooperation models such as independent research and development, cooperative development, license-in, and in-depth incubation. Fosun Pharma improves the research and clinical development capabilities of FIC (First-in-class) and BIC (Best-in-class) new drugs as well as accelerates the R&D and launch of innovative technologies and products.
-
ESG
*data from: Fosun Pharma 2024 ESG and Sustainability Report

- 复星医药集团将可持续发展纳入公司整体发展战略,重视ESG长效治理能力,建立了由董事会监管、ESG委员会实施、ESG工作小组执行的三级ESG治理架构。董事会ESG委员会负责制定并推进本集团ESG愿景、目标、策略,并向董事会提供建议;ESG工作小组负责梳理拟订重要ESG议题,拟订可持续发展量化目标并跟踪达成进度。ESG委员会和ESG工作小组致力于将ESG理念融入企业运营,提升企业可持续发展能力。
-
Investor Relations
Shanghai Fosun Pharmaceutical (Group) Co., Ltd. is a global innovation-driven pharmaceutical and healthcare industry group deep-rooted in China.

- Founded in 1994, Shanghai Fosun Pharmaceutical (Group) Co., Ltd. ("Fosun Pharma"; stock code: 600196.SH, 02196.HK) is a global innovation-driven pharmaceutical and healthcare industry group deep-rooted in China. Fosun Pharma directly operates businesses including pharmaceutical manufacturing, medical devices, medical diagnosis, and healthcare services. As a shareholder of Sinopharm Group Co., Ltd., Fosun Pharma expands its areas in the pharmaceutical distribution and retail business.
-
News
Shanghai Fosun Pharmaceutical (Group) Co., Ltd. is a global innovation-driven pharmaceutical and healthcare industry group deep-rooted in China.

- Founded in 1994, Shanghai Fosun Pharmaceutical (Group) Co., Ltd. ("Fosun Pharma"; stock code: 600196. SH, 02196. HK) is a global innovation-driven pharmaceutical and healthcare industry group deep-rooted in China. Fosun Pharma directly operates businesses including pharmaceutical manufacturing, medical devices, medical diagnosis, and healthcare services. As a shareholder of Sinopharm Co., Ltd., Fosun Pharma expands its areas in the pharmaceutical distribution and retail business.
-
Careers
Some top talent with strong entrepreneurship gathers here with us.

- We are looking for people that recognize and practice our cultural values, and can learn fast to create continuous value; possess profound professional knowledge of the industry, a wide vision of different regions, and global-level expertise in a certain field. We will also be committed to cultivating international top talent with outstanding performance and great potential.