Our Commitment
Our Strategy
-
 Stable
Stable Continuously conduct quality system evaluations to enhance compliance depth and strengthen the growth of quality capabilities and culture.
-
 Mature
Mature Continuously improve the full lifecycle quality management system, optimize quality management platforms, and advance international quality management practices.
-
 Efficient
Efficient Continuously enhance digital information systems and implement quality talent development programs.
Our Initiatives
- Production Quality Management System
- Quality Culture
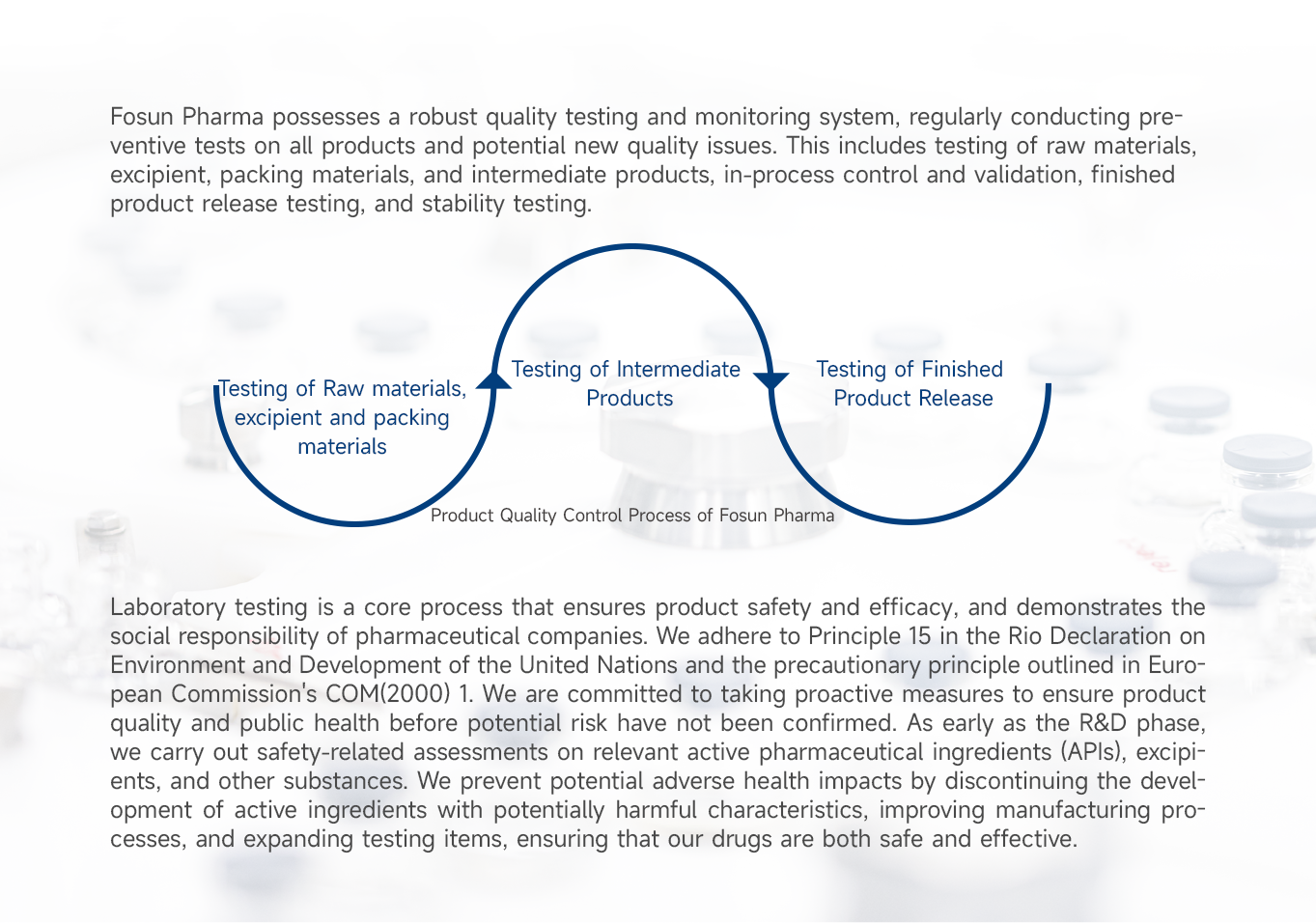
- Quality Testing
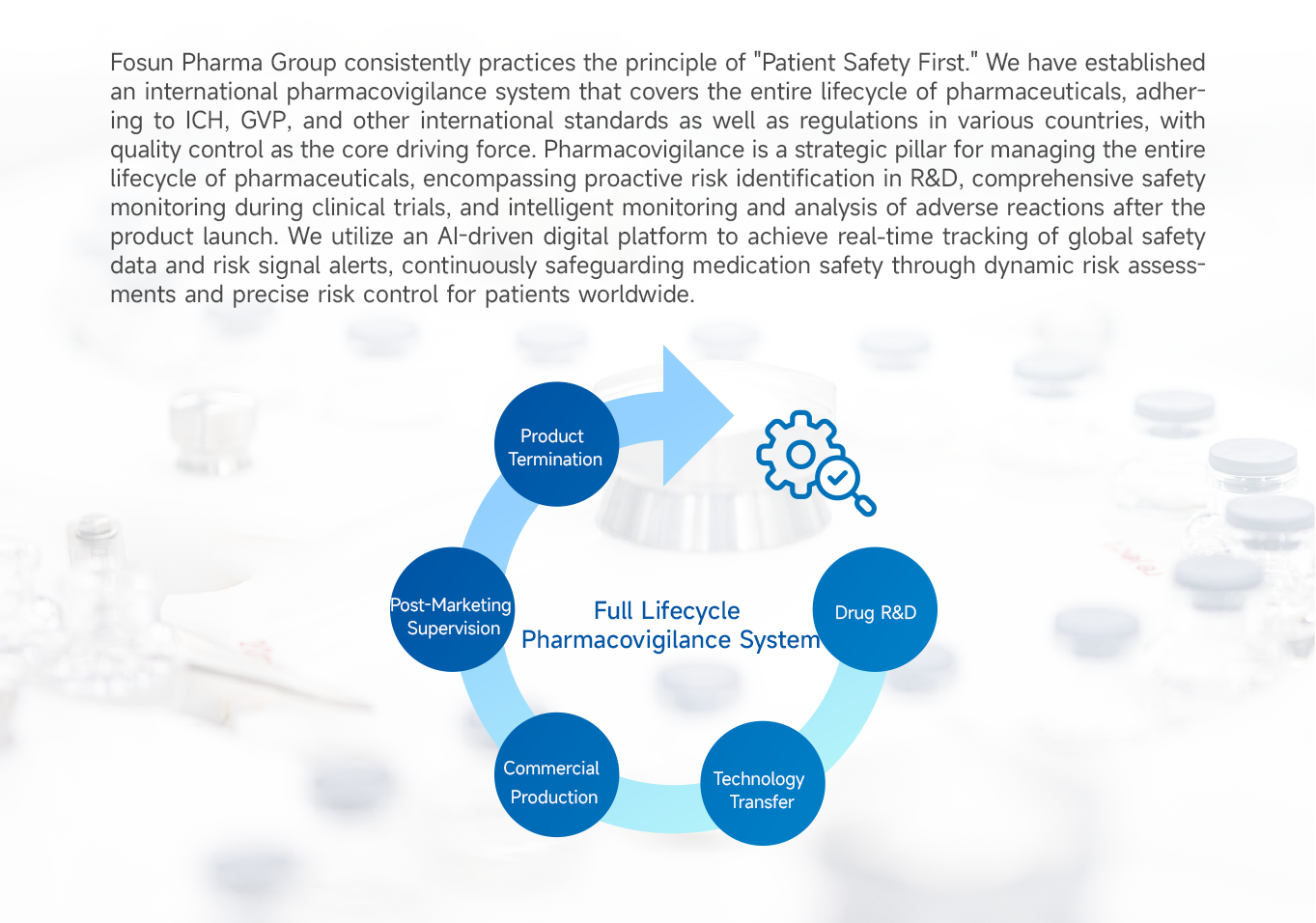
- Full Lifecycle Pharmacovigilance System
Our Achievements
-

10
10 production lines have successfully passed GMP compliance inspections conducted by the U.S. FDA, the European Union, and the World Health Organization (WHO).
-

15
15 APIs have passed FDA and WHO GMP compliance inspections.
-

>99%
The right first time for drug products exceeds 99%
-

100%
the pass rate for market sampling inspections is 100%
-

100%
Pharmaceutical subsidiaries comply 100% with GMP standards
-

93%
ISO 9001/13485 Certified Manufacturing Subsidiaries
-

100%
Quality training has been delivered to 100% of our employees
-

93hours per year per person
The average annual quality training for operation personnel is 93 hours per year per person
*As of May 31, 2025